Change in Internal Energy of an Ideal Gas
This is also called a Joule expansion. If the gas is ideal the internal energy depends only on the temperature.

Thermodynamics Derivation Of Heat Capacity At Constant Pressure And Temperature Physics Stack Exchange
The problem is that such ideal places for geothermal energy are quite limited and as such geothermal energy is a very small portion of energy production outside of certain hotspots like Iceland.

. Oxygen or compound molecules made from a variety of atoms eg. The internal energy of systems that are more complex than an ideal gas cant be measured directly. For an ideal gas the potential energy of a gas does not depend on pressure at all.
In this equation R is the ideal gas constant in joules per mole kelvin Jmol-K and T is the temperature in kelvin. An internal combustion engine has a chamber which has fuel added to it which ignites in order to raise the temperature of the gas. The reference values for the internal energies should be specified in a way that is constrained to make this so maintaining also that the internal energies are respectively proportional to the masses of the systems.
The change in internal change of gaseous reaction is to assume ideal gas behaviour and constant temperature. Gas is one of the four fundamental states of matter the others being solid liquid and plasma. It is simply equal to the thermal energy of the gas.
The internal energy of an ideal gas is therefore directly proportional to the temperature of the gas. The formula can be given as ΔU ΔH-Δ PV ΔU ΔH-Δ nRT ΔU ΔH-RTΔn Here. Proton exchange membrane fuel cell PEMFC is considered as a promising green power source for fuel cell vehicles FCV due to its high energy efficiency and power density In recent years the commercial FCVs with fuel cell power output over 100 kW are launched for mass production including the Toyota Mirai 2014 and 2021 Honda Clarity Fuel Cell 2016 and.
When sand is removed from the piston one grain at a time the gas expands adiabatically and quasi-statically in the insulated vessel. Hence it is an extensive property. The internal energy of ideal gases is a function of temperature only.
A pure gas may be made up of individual atoms eg. Raising the temperature of a gas increases the pressure that makes the gas want to expand. Carbon dioxideA gas mixture such as air contains a variety of pure gases.
The internal energy depends only upon the quantity of the substance contained in the system. If you let the canister expel its contents as a well collimated stream with negligible pressure that stream will be moving at a speed equal to the thermal velocity of the starting contents. Internal combustion heat engines can be understood by thinking carefully about the ideal gas law.
The change in internal energy for the formation of one mole of CO at the given temperature and pressure has to be given. E sys 3 2 RT. There are enhanceddeep geothermal technologies that can drill down miles into the Earths crust to tap into heat almost anywhere but those are.
For concision in this article the term ideal material is used to refer to either an ideal gas mixture or an ideal solution. A noble gas like neon elemental molecules made from one type of atom eg. Therefore when an ideal gas expands freely its temperature does not change.
Hence in isothermal processes as the temperature remains constant there is no change in the internal energy of an ideal gas ie rmU.

Ideal Gas And Kinetic Gas Theory Boltzmann Constant Thermodynamics Physics Lessons Arrow Of Time
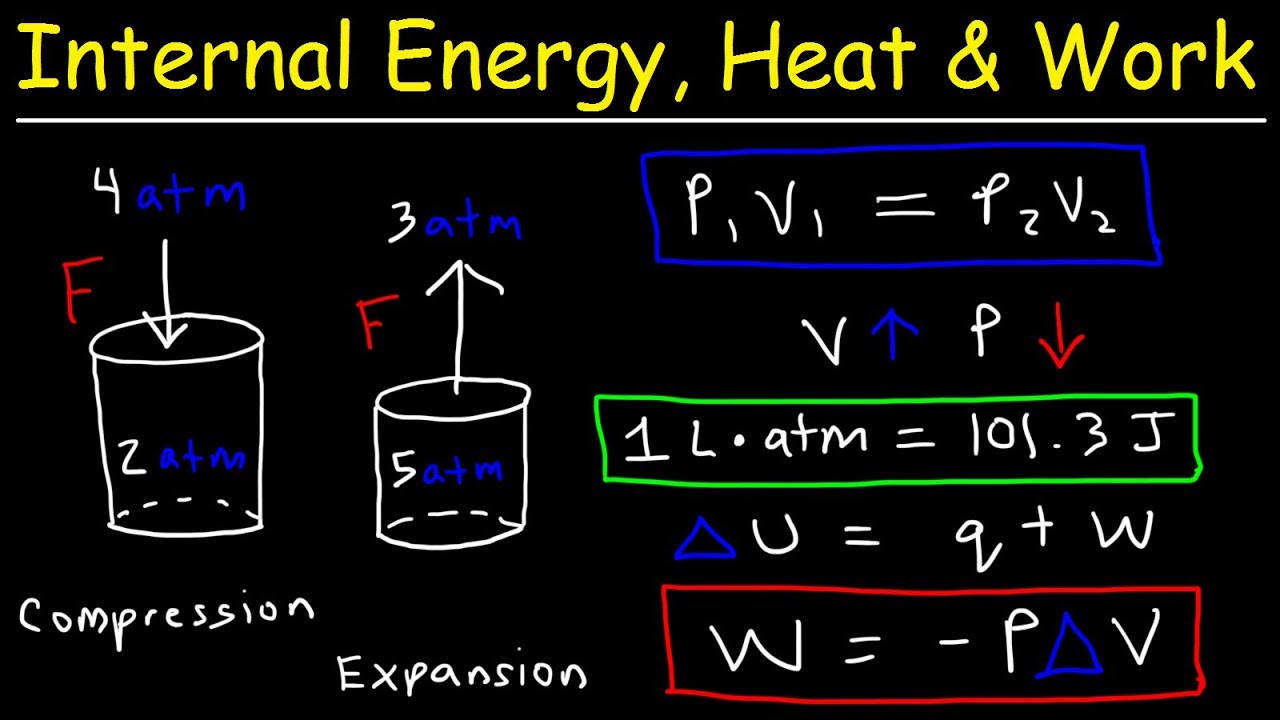
Internal Energy Heat And Work Thermodynamics Pressure Volume Chemistry Problems Youtube

Question 10 3 Chapter Ten Thermodynamics
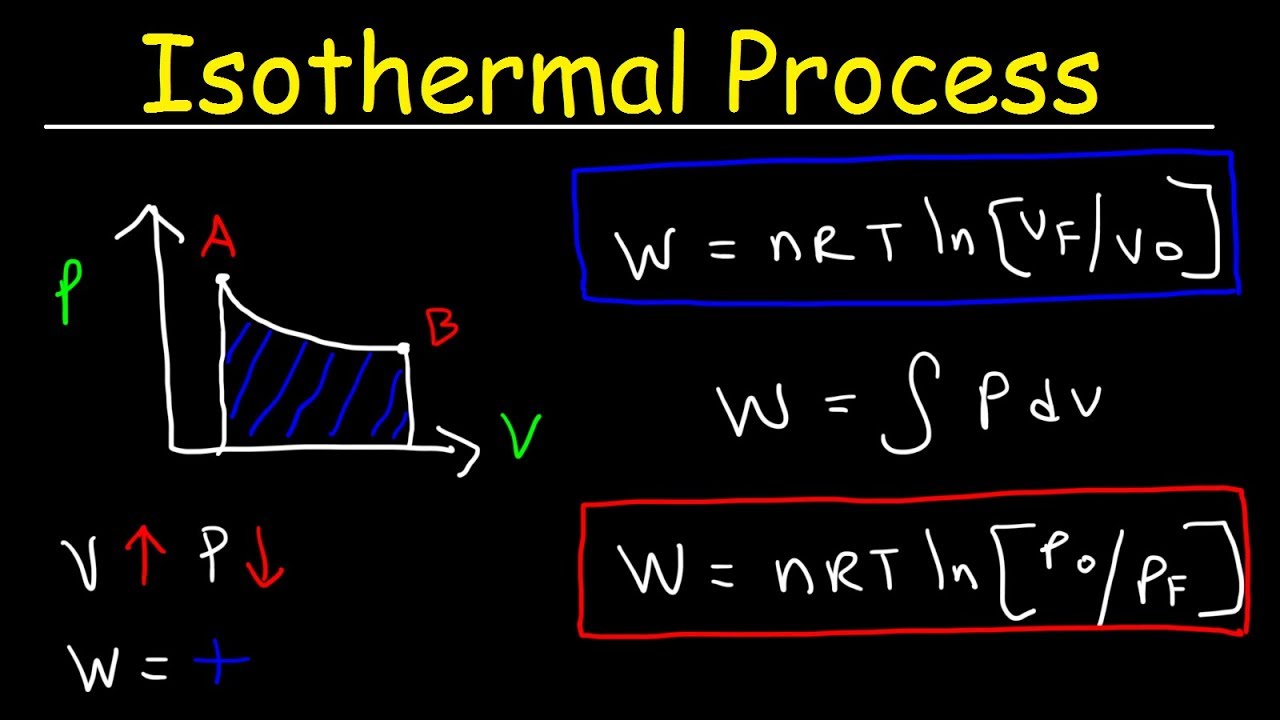
Isothermal Process Thermodynamics Work Heat Internal Energy Pv Diagrams Youtube
No comments for "Change in Internal Energy of an Ideal Gas"
Post a Comment